
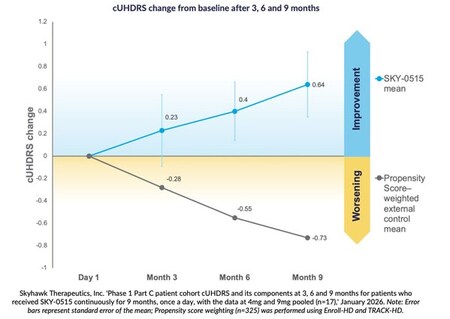
in the Composite Unified Huntington’s Disaster Rating Scale based on propensity score weighting.,The average improvement from baseline in the nine-month findings in this study was +0.64 points, compared to the expected index value of -0.73 points over a nine-month natural history for symptomatic patients.
Skyhawk also announced the global expansion of the SKY-0515 Phase II FALCON-HD trial. Skyhawk has now administered the drug to more than 90 patients.
BOSTON, Jan. 28, 2026 /PRNewswire/ — A clinical-stage biotechnology company focused on small molecule drug discovery that titrates critical RNAs.Skyhawk Therapeutics, Inc.Today, we announced that a nine-month interim analysis of Nintendo’s experimental treatment drug SKY-0515 for Huntington’s disease (HD) showed positive results.
 Skyhawk Therapeutics, Inc.
Skyhawk Therapeutics, Inc.
Administration of SKY-0515 revealed a dose-dependent effect, with blood mHTT protein decreasing by 62% and PMS1 mRNA decreasing by 26% after administration of 9 mg. PMS1 is an element that determines somatic CAG repeat expansion and HD pathology.
In the Part C patient cohort of the SKY-0515 Phase I clinical trial, patients treated with SKY-0515 demonstrated an average improvement from baseline on the Unified Huntington’s Disease Rating Scale (cUHDRS) at 3, 6, and 9 months. The predicted improvement in cUHDRS at 9 months in symptomatic patients based on propensity score weighting using Enroll-HD and TRACK-HD was -0.73 points, compared to an improvement of +0.64 points in the pooled analysis at 9 months in this study.
Ed City Wild, Professor of Neurology at University College London, said: “The safety and early efficacy data from the Phase I Part C study in patients treated with SKY-0515 showed that cUHDR “SKY-0515 has been demonstrated to reduce mHTT protein to a greater extent than any therapeutic investigational drug tested in patients to date, and clinical and biomarker data indicate that SKY-0515’s ability to reduce mHTT and PMS1 makes it a powerful combination in the treatment of these two core pathogenic mechanisms. “This will be tested in the LCON-HD trial and is expected to have a meaningful impact, making orally administered huntingtin protein-reducing treatments like SKY-0515 a true game-changer for HD patients around the world.”
Skyhawk “The goal of the Phase I study was to establish safety and biomarker efficacy,” said Sergey Paushkin, Head of Research and Development, Therapeutics. The improvement in this study compared to the worsening of patient scores in natural history data marks an important milestone for SKY-0515 and highlights Skyhawk’s superior platform to deliver first-in-class small molecules for serious diseases for which there are no approved disease-modifying drugs.
Huntington’s disease is a rare genetic neurodegenerative disease that is ultimately fatal. It is estimated that more than 40,000 people in the United States are affected, and approximately several hundred thousand people worldwide are affected. This is an orally administered small molecule RNA modulator developed using SKYSTAR® for therapeutic testing. SKY-0515 therapeutically reduces both HTT and PMS1 proteins.
Skyhawk also announced today the global expansion of its SKY-0515 Phase II FALCON-HD study, which is currently being conducted at 12 sites in Australia and New Zealand. Skyhawk has currently administered SKY-0515 to more than 90 patients.
SKY-0515 is the first Skyhawk drug in clinical trials.
By the end of 2027, Skyhawk plans to advance to the clinic with additional small molecule medicines to treat rare neurological diseases for which no approved disease-modifying drugs exist.
About the Phase I clinical trial of SKY-0515
The SKY-0515 Phase I clinical trial is a first-in-human study designed to evaluate the safety, tolerability, pharmacokinetics, and pharmacodynamics of SKY-0515 in healthy volunteers and patients with early-stage Huntington’s disease (HD). The exam will be conducted in three parts. Parts A and B evaluate SKY-0515 in healthy volunteers. Part C is a double-blind, placebo-controlled, parallel-design study in early-stage HD patients (HD-ISS stages 1, 2, or mild stage 3). SKY-0515 and placebo will be administered at two dose levels for 84 days, followed by 12 months of active treatment in which all participants receive either a low or high dose of SKY-0515 in a blinded manner. Evaluation of mRNA. The first patient administration in Part C of SKY-0515 was performed in January 2025. Phase 1C enrollment for the SKY-0515 study has been completed.
About SKY-0515 Phase II FALCON-HD clinical trial
Falcon-HD (NCT06873334) is a Phase II, randomized, double-blind, placebo-controlled, dose-finding study evaluating the pharmacodynamics, safety, and efficacy of SKY-0515 in 120 patients with early stage 2 and stage 3 HD at 12 sites in Australia and New Zealand, and 400 patients with early stage 2 and stage 3 HD, both at more than 40 sites worldwide. Eligible patients will receive SKY-0 515 orally once daily at one of three doses or a placebo. The treatment period is 12 months or more. The purpose of this study is to evaluate the potential of SKY-0515 to modulate RNA pricing and reduce mHTT and PMS1 proteins, which are involved in the pathology of Huntington’s disease. For more information about FALCON-HD, including participating sites and eligibility criteria, please visitClinicalTrials.govandwww.FALCON-HD.comYou can check it here.
About Skyhawk Therapeutics
Skyhawk Therapeutics is a clinical-stage biotechnology company. Using our unique platform, SKYSTAR®, we are discovering and developing small molecule RNA modulators for diseases that are considered to be particularly incurable in the world.www.skyhawktx.comPlease take a look.
Skyhawk contact information
maura mccarthy
Head of Corporate Development
maura@skyhawktx.com
photograph- https://mma.prnasia.com/media2/2870056/Skyhawk_Therapeutics_9_MONTH_DATA_Graph.jpg?p=medium600
logo- https://mma.prnasia.com/media2/710814/Skyhawk_Therapeutics_Logo.jpg?p=medium600
(Japanese release: provided by client)
PR Newswire Asia Co., Ltd.

PR News Wire
Founded in 1954, it is the world’s first American public relations news agency. Our distribution network covers the entire world. As a subsidiary of Cision Ltd., Cision cloud-based communications products, the world’s largest multichannel, multicultural content dissemination network, offer workflow tools and platforms that power the stories of organizations across the globe.www.prnasia.com