Transferrin is a glycoprotein with a molecular weight of 80 kDa that is synthesized in the liver.
Each transferrin molecule can bind up to two iron atoms for distribution into cells. The half-life of transferrin in humans is approximately 8 days, whereas iron delivery via the transferrin/transferrin receptor cycle is completed in approximately 5 to 20 minutes, depending on the cell type.
This means that each transferrin molecule can facilitate hundreds of cycles of iron binding and iron delivery to cells during its lifetime.1
Figure 1. Transferrin 3D structure (PDB, RCSB)2. Image credit: HORIBA Scientific
Measuring transferrin iron levels in serum is an essential part of assessing iron metabolism. Abnormal transferrin levels in serum are associated with a variety of diseases, including certain cancers and other inflammatory syndromes.3
Duetta is a highly sensitive spectrofluorometer that can be used to measure very low concentrations of transferrin. The Duetta spectrofluorometer offers important advantages in protein analysis by simultaneously integrating both fluorescence and absorbance measurements.

Image credit: HORIBA Scientific
In a comparative study, Horiba evaluated fluorescence results obtained using Duetta and absorbance results from a dedicated low-volume benchtop spectrophotometer.
Method and results
For fluorescence and absorbance measurements, transferrin was prepared in 10 mM PBS at concentrations ranging from 50 μg/mL to 0.75 μg/mL. The same buffer without protein served as a blank.
The excitation wavelength was set to 250 nm, and the emission was collected from 265 nm to 550 nm using a 2 s integration time and a 10 nm slit on the CCD.
Transferrin solutions from 0.75 μg/mL to 50 μg/mL were measured in absorbance mode. A comparison of the absorbance spectra obtained at the highest concentration from both systems is shown below.
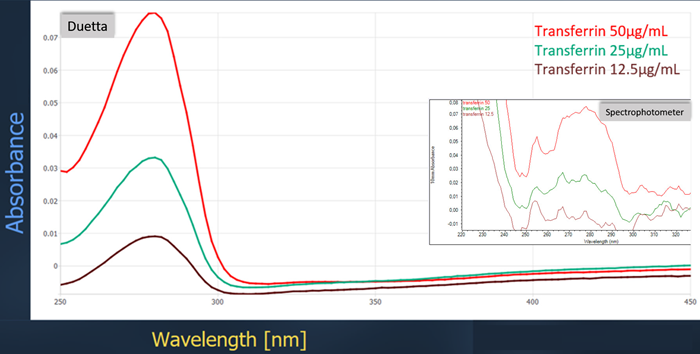
Figure 2. Comparison of absorbance spectra between instruments. Image credit: HORIBA Scientific
The obtained spectra show noticeable differences. The Duetta spectrofluorometer detected 12.5 μg/mL of transferrin, and the signal increased linearly with concentration. Comparative data from the spectrophotometer showed a clearly noisy curve with a detection limit of approximately 25 µg/mL.
Figure 3 shows the resulting fluorescence spectra overlaid.
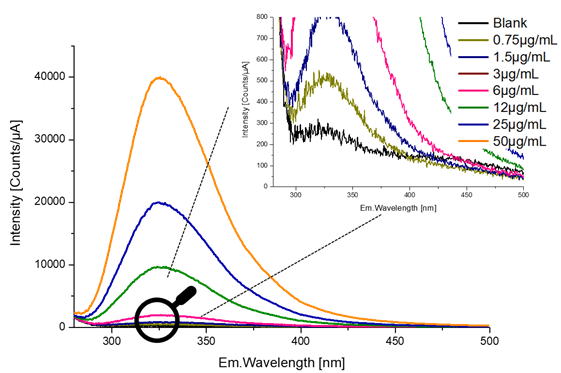
Figure 3. Fluorescence emission spectra of transferrin at increasing concentrations (0.75 μg/ml to 50 μg/ml). Image credit: HORIBA Scientific
Zooming in on the lowest concentration shows that the detection limit is approximately 0.75 µg/mL.
Although Duetta is twice as sensitive in measuring protein concentration by UV absorption, its superior performance is further demonstrated by fluorescence, as shown in the fluorescence profile in Figure 4.
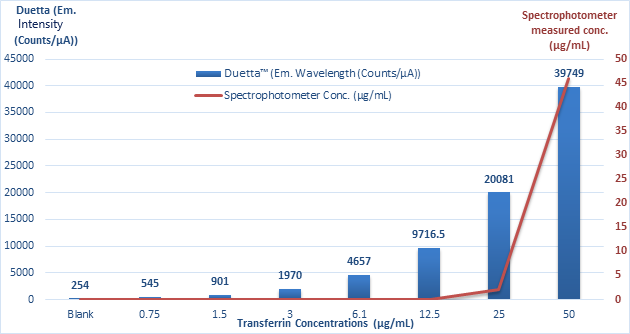
Figure 4. Comparison of transferrin concentration measurements obtained with a spectrophotometer and a Duetta instrument. Image credit: HORIBA Scientific
The graph shows that the detection limit for transferrin using Duetta is approximately 0.75 µg/mL. The amplitude of the signal is directly proportional to the measured concentration.
In contrast, the lowest concentration detected by the spectrophotometer is 25 μg/mL, making it 33 times less sensitive than Duetta. This further demonstrates the sensitivity of fluorescence spectroscopy.
conclusion
Fluorescence spectroscopy has numerous applications in the life sciences, from cell biology to genomics. For protein characterization, the intrinsic fluorescence of certain amino acids (tryptophan, tyrosine, and phenylalanine) provides a valuable capability to study proteins without the need for labeling.
The work presented here demonstrates the high sensitivity of Duetta with fluorescence measurements compared to traditional UV absorbance approaches.
Duetta has a detection limit of approximately 25 µg/mL using absorbance, but can successfully detect 0.75 µg/mL transferrin using fluorescence. This detection limit can be enhanced by optimizing certain parameters, such as increasing the integration time, widening the slit, or increasing the emission increments (binning).
Duetta can measure both UV-visible absorbance and fluorescence spectra and exhibits higher sensitivity than other benchtop spectrophotometers designed specifically for absorbance measurements.
The Duetta spectrofluorometer’s optimized optical layout and dual-purpose design make it ideal for protein spectroscopy and analysis of low concentration solutions.
References and further reading
- Choi, S. (2018). Encyclopedia of signal transduction molecules. https://doi.org/10.1007/978-3-319-67199-4.
- McGillivray, RTA; others. (1998). Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal structural changes associated with iron release†,‡. biochemistry37(22), pp. 7919–7928. https://doi.org/10.1021/bi980355j.
- Gkouvatsos, K., Papanikolaou, G., and Pantopoulos, K. (2012). Regulation of iron transport and the role of transferrin. Biochimica et Biophysica Acta (BBA) – General subjects1820(3), pp. 188–202. https://doi.org/10.1016/j.bbagen.2011.10.013.
About Horiba 
Horibais headquartered in the United States and offers a wide range of instruments and solutions for a wide range of scientific research and development and QC measurement applications. HORIBA is a world leader in OEM spectroscopy, elemental analysis, fluorescence (including PTI brands), forensics, GDS, ICP, particle characterization, Raman, spectroscopic ellipsometry, sulfur in oil and water quality measurements, and XRF. Our equipment is used in universities and industry around the world. Proven quality and reliable performance have established widespread trust in the HORIBA brand.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources with which we have an existing commercial relationship, provided the content adds value to News-Medical.Net’s core editorial philosophy of educating and informing site visitors interested in medical research, science, medical devices, and treatments.