The Navitar valve, a novel intraannular self-expanding transcatheter heart valve (THV) for the treatment of native aortic stenosis (AS) in high-risk or extreme-risk patients, has shown favorable 30-day results and reduced permanent pacemaker implantation (PPI) rates in surgically experienced sites. According to research Published on January 14th JACC: Cardiovascular Intervention.
Dr. Santiago Garcia, FACC, Vinod H. Thulani, MD, FACCwere treated with the Navitor valve between January 2023 and December 2024; STS/ACC TVT Registry. The study’s primary outcome was 30-day post-surgery all-cause mortality or stroke combined.
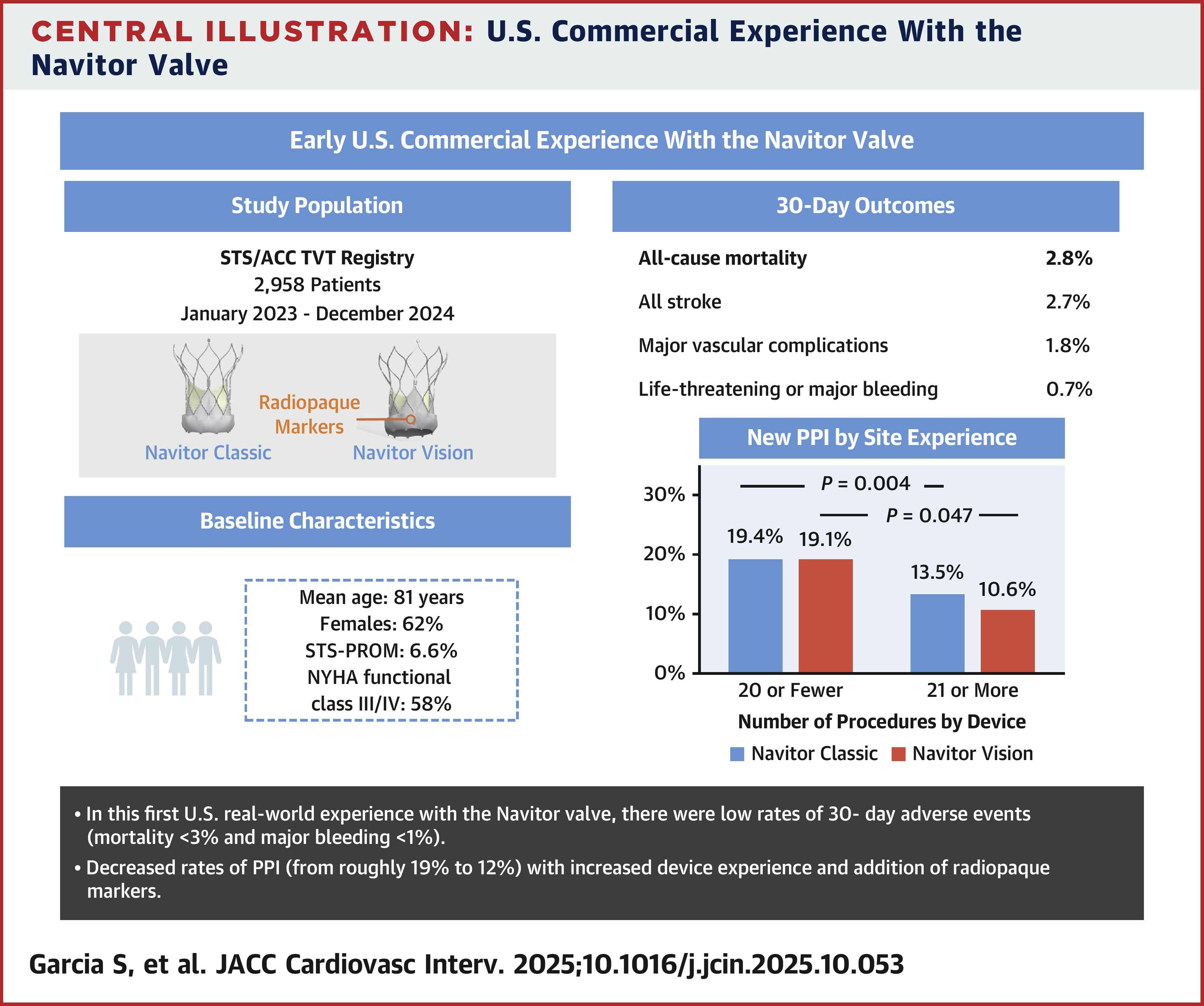
Technical success was observed in 97.9% of cases, and in-hospital death occurred in 1.3% of cases. The composite primary outcome occurred in 5.2% (mortality 2.8%, stroke 2.7%). Additional outcomes after 30 days were vascular complications in 1.8% of patients and PPI in 17.8%.
A secondary analysis of case volume by center found that centers with more than 20 surgical cases in Navitor Classic and Vision were less likely to require PPI after TAVR (Classic: 19.4% vs. 13.5%, p=0.0039; Vision: 19.1% vs. 10.6%, p=0.047).
The authors describe the commercial introduction of Navitor in the United States as “safe and effective” and highlight how this valve design may benefit young TAVR patients in the future.
“As TAVR expands to younger patients with low surgical risk, lifelong management of AS patients must be considered, including hemodynamic performance, maintenance of coronary access, valve durability, and maintenance of future THV-in-THV options,” the authors note. “…ongoing studies in low-risk patients will shed light on long-term clinical outcomes and valve performance.”