Results showed a good safety profile and improved function in patients with severe tricuspid regurgitation, including those with decreased right ventricular function.
Yoknem, Israel, January 21, 2026 /PRNewswire/ — Trisol Medical, a clinical-stage medical device company developing a transcatheter tricuspid valve replacement system, today announced positive results from an FDA-approved U.S. early feasibility study evaluating the Trisol transcatheter tricuspid valve in patients with severe to severe tricuspid regurgitation.
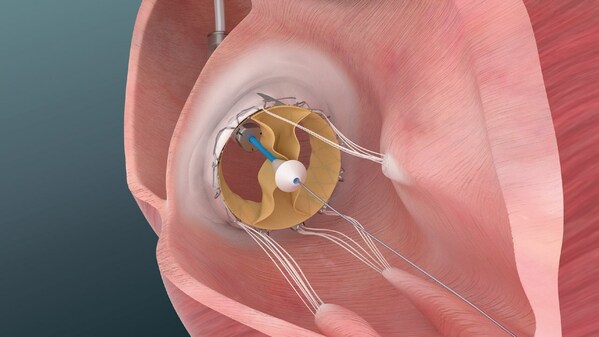
Diagram of Trisol Medical’s transcatheter tricuspid valve replacement system. It is being used in the company’s early U.S. feasibility study in patients with severe to extreme tricuspid regurgitation.
Trisol’s Transcatheter Tricuspid Valve Replacement (TTVR) System is designed to treat severe to severe tricuspid regurgitation (TR), a condition in which the tricuspid valve does not close properly and blood flows backwards from the right ventricle into the right atrium. Patients with this condition often have limited treatment options.
To date, 22 patients with severe to severe TR who were considered at high risk for traditional surgery have been treated using the transjugular (TJ) access approach at major centers in the United States, including Piedmont Heart Institute, Vanderbilt University Medical Center, University of Virginia Health System, Columbia University Medical Center, and Cedars-Sinai Medical Center. Enrollment in the TJ cohort is now complete, and ongoing enrollment studies are underway using Trisol’s newly developed transfemoral access route.
Main research results
- Safety: The need for a permanent pacemaker is less than 5% at 30-day follow-up.
- effect: Significant reduction in TR after transplantation.
- Functional outcome: At 30-day and 12-month follow-up, significant improvements were observed in quality of life (KCCQ), heart failure symptoms (NYHA class), 6-minute walking distance, right ventricular function, and cardiac output.
- technical performance: Device deployment was successful and no migration occurred.
Importantly, these encouraging results include patients with reduced right ventricular function, a large, high-risk subgroup that is associated with poor outcomes and is underserved by existing and emerging therapeutic approaches. Initial clinical experience with emerging therapies reported to date highlights an unmet need in this patient population and suggests that the Trisol valve may help address this critical gap.
Dr. Pradeep Yadav commented: “Trisol brings several new features, from ease of use to recaptureable anchors, a wide size range, lower pacemaker rates, and performance in a failing right ventricle. We are very excited to explore the next steps with its transfemoral delivery system and pivotal trials.”
Ron Davidson, CEO of Trisol Medical, said, “We are excited about these positive results, which further demonstrate the potential of our best-in-class technology to improve the care of patients with severe TR. We would like to thank our clinical researchers and the entire clinical team for their dedication and outstanding patient care.”
Dr. Simon Eckhouse, chairman of Trisol, added, “Millions of Americans suffer from TR, and despite recent advances, effective treatment options for patients with severe TR remain limited. The Trisol valve was developed to address this important unmet need and has the potential to redefine the standard of care.”
About Trisol Medical
Trisol Medical Ltd. is a clinical-stage medical device company specializing in the development of new TTVRs. Founded within the Alon MedTech Ventures incubator, Trisol is dedicated to advancing minimally invasive heart valve treatments that provide safer alternatives to traditional surgery. See below for more information. www.trisol-medical.com.
Media contact:
ron davidson
CEO
rond@trisol-medical.com